which particles have approximately the same size and mass|Printable Chemistry Quiz : Clark Protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000 times as massive as an electron). The . Powerball is an American lottery game offered by 45 states, the District of Columbia, Puerto Rico and the U.S. Virgin Islands, and overseen by the Multi-State Lottery Association (MUSL), which also manages other large jackpot games such as the Mega Millions.Drawings are held three times weekly on Mondays, Wednesdays, and .
PH0 · Which subatomic particles have approximately the same mass?
PH1 · Which subatomic particles have approximately the same mass?
PH2 · Which particles have approximately the same size and mass?
PH3 · Which particles have approximately the same size and mass as
PH4 · What two particles have approximately the same mass?
PH5 · Subatomic Particles You Should Know
PH6 · Printable Chemistry Quiz
PH7 · Atom Quiz
PH8 · 4.4: The Properties of Protons, Neutrons, and Electrons
PH9 · 2.1: Atoms: Their Composition and Structure
PH10 · 1.8: Subatomic Particles
This article is about Foop in the original series. For information on him in A New Wish, see Irep. Hello Clarice, I mean mother.Foop's first spoken words Foop Anti-Cosma-Anti-Fairywinkle is the Anti-Fairy counterpart and side effect of Poof and the son of Anti-Cosmo and Anti-Wanda. He is supposedly the first Anti-Fairy baby born in thousands of years, .
which particles have approximately the same size and mass*******Which particles have approximately the same size and mass as each other? (a) neutrons and electrons. (b) electrons and protons. (c) protons and neutrons. (d) none - they are all .Protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000 times as massive as an electron). The . Protons and neutrons have nearly the same size and mass. Electrons are so much smaller and lighter they basically don't even affect the mass of an atom.The masses of the subatomic particles are given below - Subatomic particle Mass (kg) Proton 1.6726 × 10 − 27 Neutron 1.6749 × 10 − 27 Electron 9.1093 × 10 − 31 The mass . The nuclear particles, protons and neutrons, are conceived to have a positive electronic charge, and a neutral charge respectively. The protonic mass is .
Neutrons have approximately the same mass as protons but no charge. They are electrically neutral. The mass of a proton or a neutron is about 1836 times greater than the mass of an electron.Neutrons have approximately the same mass as protons but no charge. They are electrically neutral. The mass of a proton or a neutron is about 1836 times greater than . The atomic nucleus consists of two subatomic particles that are bonded together by the strong nuclear force. One of these particles is the proton. The other is the neutron. Neutrons are approximately the . 1 Answer. The nucular particles: protons and neutrons. The nuclear particles, protons and neutrons, are conceived to have a positive electronic charge, and a neutral charge respectively. The protonic mass is 1.672621898(21) × 10−27 ⋅ kg. And the neutron mass is 1.674927471(21) × 10−27 ⋅ kg, so to a first approx. these masses are .The masses of the subatomic particles are given below -. Subatomic particle Mass (kg) Proton 1.6726 × 10−27 Neutron 1.6749 × 10−27 Electron 9.1093 × 10−31. The mass of a proton is nearly same as the mass of a neutron, whereas the mass of an electron is nearly ( 1 1836) times that of the proton. Suggest Corrections.
Answer and Explanation: 1. Become a Study.com member to unlock this answer! Create your account. View this answer. The two subatomic particles that have the same mass are protons and neutrons. Neutrons and protons each have an atomic mass of about one atomic mass. See full answer below.
The subatomic particles which are having approximately the same mass are neutrons and protons. Thus, option c is correct.. What are subatomic particles? An atom is made of subatomic particles namely neutrons, protons and electrons.The neutrons and protons are located inside the nucleus whereas, the electrons are revolving around .
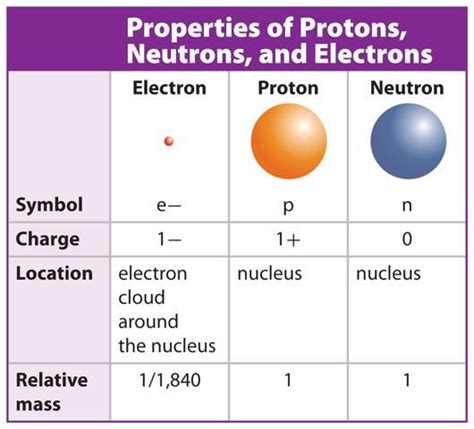
The subatomic particles with approximately the same size and mass are protons and neutrons. They have a similar size, with a diameter of about 1 femtometer (10^-15 meters), and a mass of approximately 1 atomic mass unit (amu). On the other hand, electrons, which have a much smaller mass, are estimated to be about 10,000 times .which particles have approximately the same size and mass The subatomic particles with approximately the same size and mass are protons and neutrons. They have a similar size, with a diameter of about 1 femtometer (10^-15 meters), and a mass of approximately 1 atomic mass unit (amu). On the other hand, electrons, which have a much smaller mass, are estimated to be about 10,000 times .Printable Chemistry Quiz The subatomic particles with approximately the same size and mass are protons and neutrons. They have a similar size, with a diameter of about 1 femtometer (10^-15 meters), and a mass of approximately 1 atomic mass unit (amu). On the other hand, electrons, which have a much smaller mass, are estimated to be about 10,000 times .When the numbers of these subatomic particles are not equal, the atom is electrically charged and is called an ion. The charge of an atom is defined as follows: Atomic charge = number of protons − number of electrons (1.8.1) (1.8.1) Atomic charge = number of protons − number of electrons.Study with Quizlet and memorize flashcards containing terms like what subatomic particles have approximately the same mass?, The pure form of matter that cannot be broken down into a simpler form is a(n), 8) The acidity or alkalinity of a solution can be measured in terms of and more.
We would like to show you a description here but the site won’t allow us.
The masses of the subatomic particles are given below. Subatomic particle Mass (kg) Proton 1.6726 × 10 − 27 Neutron 1.6749 × 10 − 27 Electron 9.1093 × 10 − 31 The mass of a proton is nearly same as the mass of a neutron, whereas the mass of an electron is nearly (1 1836) times that of the proton.which particles have approximately the same size and mass Printable Chemistry Quiz The three particles that make up an atom are protons, neutrons and electrons. However, electrons have a mass of 1/1837 of that of a proton or a neutron, which is a negliglible mass and therefore does not contribute to the mass of an atom. Therefore, the mass number of an atom is the sum of the number of protons and the number of .
VIDEO ANSWER: Atomic theory is a scientific theory of nature. The state that matter is in is made up of atoms. The electrons, protons and neutrons are what make up atoms. The electrons have a negative charge. Charge electrons are so. Protons and neutrons have approximately the same size and mass as each other. Protons have a positive charge and are located in the nucleus of an atom, while neutrons have no charge and are also .

The answer is (4) neutron and proton. The electron has very little mass and the neutron and proton have approximately the same mass. The positron has approximately the same mass as electron. The beta particle is electron. And the alpha particle is the nucleus of He which is about the same mass as 4 protons. inside the nucleus. Table 4.5.1 4.5. 1 gives the properties and locations of electrons, protons, and neutrons. The third column shows the masses of the three subatomic particles in "atomic mass units." An atomic mass unit (amu amu) is defined as one-twelfth of the mass of a carbon-12 atom.The correct answer is option a. The subatomic particles having approximately similar masses are neutrons and protons and the values of their masses respectively are 1.6749 10-27 kg
W hen the government launched a call for evidence on gambling in December 2020, anti-gambling groups were straight out of the blocks with a series of policy demands. They wanted a ban on gambling advertising, a ban on gambling sponsorship, a limit on how much people could spend on gambling each month, a ban on VIP .
which particles have approximately the same size and mass|Printable Chemistry Quiz